" It is a region that forms when electrons from different atoms interact with each other. The electrons that participate in chemical bonds are the valence electrons, which are the electrons found in an atom's outermost shell. When two atoms approach each other these outer electrons interact. Electrons repel each other, yet they are attracted to the protons within atoms. The interplay of forces results in some atoms forming bonds with each other and sticking together ".
What is exactlly Chemical Bonding???
The world around us is made up of tiny units of matter called atoms. How these atoms stick together to form substances is called chemical bonding.
The Outer Shell
All atoms would like to have a full outer shell, but the only elements to naturally have a full outer shell are the noble gases to the right of the periodic table. As a result, when atoms without full outer shells come into contact with other atoms, they tend to want to give up or gain electrons.
Valence Electrons
The valence electrons are the number of electrons an atom must lose or gain to have a full outer shell.
Atoms with a relatively empty outer shell will want to give up electrons. For example, if an atom has 1 electron out of a possible 8 in its outer shell, it will want to give up that electron so its outer shell is now full.
Atoms with a relatively full outer shell will want to gain electrons to fill up the outer shell. For example, an atom with 6 of 8 electrons in its outer shell will try to gain 2 electrons so its outer shell is full.
What is exactlly Chemical Bonding???
The world around us is made up of tiny units of matter called atoms. How these atoms stick together to form substances is called chemical bonding.
| About Atoms Each element has its own unique atom made up of a specific number of protons in its nucleus called the atomic number. Each atom also has the same number of electrons as it has protons. Electron Shells The electrons orbit around the nucleus of the atom. They stay in layers called shells. Each shell can only contain a certain number of electrons: the first layer can hold two electrons, the second layer eight electrons, the third layer eighteen electrons, etc. | 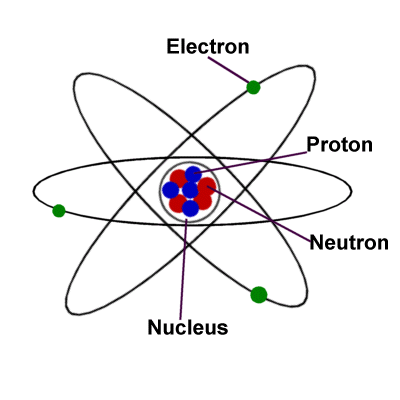 |
All atoms would like to have a full outer shell, but the only elements to naturally have a full outer shell are the noble gases to the right of the periodic table. As a result, when atoms without full outer shells come into contact with other atoms, they tend to want to give up or gain electrons.
Valence Electrons
The valence electrons are the number of electrons an atom must lose or gain to have a full outer shell.
Atoms with a relatively empty outer shell will want to give up electrons. For example, if an atom has 1 electron out of a possible 8 in its outer shell, it will want to give up that electron so its outer shell is now full.
Atoms with a relatively full outer shell will want to gain electrons to fill up the outer shell. For example, an atom with 6 of 8 electrons in its outer shell will try to gain 2 electrons so its outer shell is full.
 RSS Feed
RSS Feed Twitter
Twitter




 Shah Alam
Shah Alam
0 comments:
Post a Comment